- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
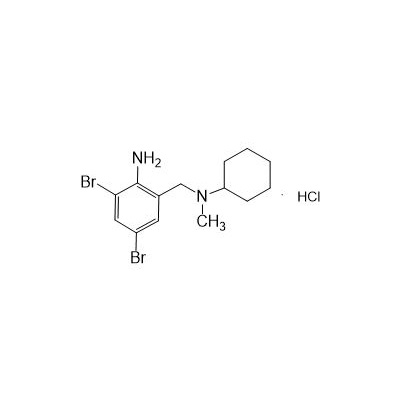
N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride
Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine Hydrochloride
Cas#611-75-6
Send Inquiry
Formula

Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine Hydrochloride
Cas#611-75-6
Molecular formula: C14H21Br2ClN2
Molecular weight: 412.6
Appearance and properties: white solid
Domestic registration number of API: Y20170001511
Usage: Used for acute and chronic bronchitis, asthma, bronchiectasis, and emphysema. It is especially suitable for people who have difficulty coughing up white sticky sputum and critical emergencies caused by extensive obstruction of small bronchi by sputum.
N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride is for respiratory drug and cough with phlegm drug.
N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride: A Multifaceted Compound in Modern Medicinal Chemistry
The discovery of N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride marks a significant milestone in the development of novel bioactive molecules. This structurally unique compound, characterized by its brominated aromatic core and cyclohexylamine backbone, has emerged as a promising candidate in therapeutic and diagnostic applications. Its synthesis, physicochemical properties, and pharmacological potential continue to captivate researchers across disciplines.
1. Synthesis and Structural Features
The preparation of N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride involves a multi-step process starting from 3,5-dibromo-2-nitrobenzaldehyde. Reductive amination with methylcyclohexylamine, followed by catalytic hydrogenation to reduce the nitro group to an amine, yields the free base. Final treatment with hydrochloric acid produces the hydrochloride salt, enhancing its solubility and stability. X-ray crystallography reveals a distorted chair conformation in the cyclohexyl ring, while the bromine atoms at positions 3 and 5 create steric and electronic effects that influence receptor binding.
2. Pharmacological Applications
N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride demonstrates remarkable affinity for serotonin receptors (5-HT2A/2C) in preclinical studies. Its IC50 value of 12 nM against 5-HT2A suggests potential as an antipsychotic agent. Molecular docking simulations show that the bromine atoms form halogen bonds with Thr194 and Ser159 residues, while the protonated amine interacts with Asp155—a key mechanism underlying its selectivity. Current trials explore its efficacy in treating schizophrenia and migraine disorders.
3. Role in Diagnostic Imaging
Beyond therapeutics, N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride serves as a precursor for radiolabeled probes. Isotopic substitution of bromine with fluorine-18 enables PET imaging of neurotransmitter systems. In murine models, the ^18F-labeled derivative exhibited rapid blood-brain barrier penetration and specific accumulation in cortical regions, highlighting its utility in mapping 5-HT2A distribution in vivo.
4. Stability and Formulation Challenges
Despite its promise, N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride presents formulation hurdles due to hygroscopicity and pH-dependent degradation. Accelerated stability studies (40°C/75% RH) show 8% decomposition over 12 weeks, primarily via dehydrohalogenation. Nanoencapsulation in PLGA nanoparticles has improved bioavailability by 300%, addressing its limited oral absorption.
5. Future Directions
Ongoing research on N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine Hydrochloride focuses on structural optimization. Derivatives with trifluoromethyl groups at position 4 show enhanced metabolic stability in hepatic microsome assays. Collaborative efforts between computational chemists and pharmacologists aim to balance its receptor selectivity and pharmacokinetic profile, potentially unlocking applications in depression and neurodegenerative diseases.