- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Bromhexine HCl API
Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine HCl API
Cas#611-75-6
Send Inquiry
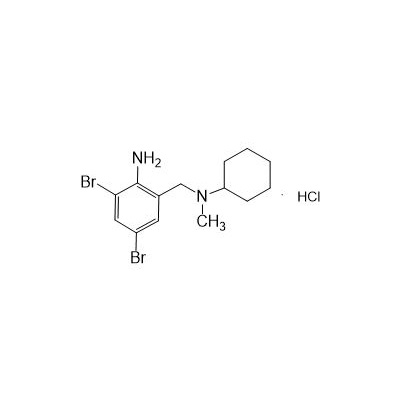
Formula

Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine HCl API
Cas#611-75-6
Molecular formula: C14H21Br2ClN2
Molecular weight: 412.6
Appearance and properties: white solid
Domestic registration number of API: Y20170001511
Usage: Used for acute and chronic bronchitis, asthma, bronchiectasis, and emphysema. It is especially suitable for people who have difficulty coughing up white sticky sputum and critical emergencies caused by extensive obstruction of small bronchi by sputum.
Bromhexine HCl API is for respiratory drug and cough with phlegm drug.
Bromhexine HCl API: A Comprehensive Overview of Its Pharmacological and Industrial Significance
Introduction
Bromhexine hydrochloride (Bromhexine HCl API) is a mucolytic agent widely recognized for its efficacy in managing respiratory disorders characterized by excessive mucus production. As an active pharmaceutical ingredient (API), Bromhexine HCl has been extensively studied and utilized in formulations ranging from oral tablets to syrups. This article explores the multifaceted aspects of Bromhexine HCl API, including its pharmacological properties, synthesis, therapeutic applications, quality control measures, and market dynamics, underscoring its importance in modern medicine.
Pharmacological Mechanism
Bromhexine HCl API exerts its therapeutic effects by depolymerizing mucopolysaccharide fibers in bronchial secretions, thereby reducing mucus viscosity and enhancing expectoration. The compound acts as a secretolytic agent, stimulating the production of serous fluid in the respiratory tract, which facilitates mucus clearance. Studies have demonstrated that Bromhexine HCl API also enhances the penetration of antibiotics into lung tissue, making it a valuable adjunct in treating bacterial respiratory infections. Its dual action—mucolytic and antimicrobial synergy—positions it as a cornerstone in respiratory therapy.
Synthesis and Manufacturing
The synthesis of Bromhexine HCl API involves a multi-step chemical process starting from the precursor compound vasicine, a natural alkaloid derived from Adhatoda vasica. Key steps include bromination, hydrolysis, and subsequent salt formation with hydrochloric acid to achieve the final hydrochloride form. Manufacturers adhere to Good Manufacturing Practices (GMP) to ensure high purity (>99%) and compliance with pharmacopeial standards (e.g., USP, EP). Advanced analytical techniques, such as HPLC and NMR, are employed to validate the structural integrity and consistency of Bromhexine HCl API across production batches.
Therapeutic Applications
Clinically, Bromhexine HCl API is indicated for chronic bronchitis, asthma, and other obstructive pulmonary diseases. Its efficacy in pediatric and geriatric populations has been well-documented, with dosing adjusted based on age and renal function. Recent research also highlights its potential anti-inflammatory and antiviral properties, particularly in mitigating SARS-CoV-2-induced mucus hyperproduction. Formulations containing Bromhexine HCl API are often combined with bronchodilators or antihistamines to address comorbid respiratory symptoms, further broadening its therapeutic scope.
Quality Control and Regulatory Compliance
The global demand for Bromhexine HCl API necessitates stringent quality control protocols. Residual solvent analysis, particle size distribution, and microbial limits are rigorously monitored to meet regulatory guidelines. Stability studies under varying temperature and humidity conditions ensure the API retains its potency throughout its shelf life. Regulatory agencies, including the FDA and EMA, mandate comprehensive documentation of Bromhexine HCl API’s safety profile, including genotoxicity and pharmacokinetic data, to secure market approval.
Market Dynamics and Future Prospects
The market for Bromhexine HCl API is projected to grow at a CAGR of 4.2% from 2023 to 2030, driven by rising respiratory disease prevalence and increasing generic drug production. Asia-Pacific dominates API manufacturing, with India and China being key exporters. Innovations in sustained-release formulations and combination therapies are expected to further elevate the demand for Bromhexine HCl API. However, challenges such as raw material sourcing and environmental regulations may impact production costs.