- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
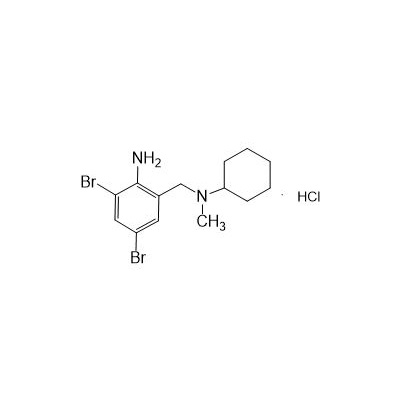
2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride
Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine Hydrochloride
Cas#611-75-6
Send Inquiry
Formula

Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine Hydrochloride
Cas#611-75-6
Molecular formula: C14H21Br2ClN2
Molecular weight: 412.6
Appearance and properties: white solid
Domestic registration number of API: Y20170001511
Usage: Used for acute and chronic bronchitis, asthma, bronchiectasis, and emphysema. It is especially suitable for people who have difficulty coughing up white sticky sputum and critical emergencies caused by extensive obstruction of small bronchi by sputum.
2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride is for respiratory drug and cough with phlegm drug.
2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride: A Multifaceted Compound in Modern Chemistry
In the realm of synthetic organic chemistry and pharmaceutical research, 2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride stands out as a structurally intricate and functionally versatile compound. Combining a brominated aromatic core with a modified cyclohexylamine backbone, this molecule exemplifies the synergy of halogenation and amine functionalization strategies. Its unique architecture and physicochemical properties have spurred interest across diverse scientific disciplines, from drug discovery to materials science.
1. Structural Insights and Chemical Significance
2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride is characterized by a 2-aminobenzyl scaffold substituted with bromine atoms at the 3- and 5-positions, linked to an N-cyclohexyl-N-methylamine group. The hydrochloride salt enhances its solubility and stability, making it suitable for experimental and industrial applications. Key structural features include:
Brominated Aromatic Ring: The electron-withdrawing bromine atoms increase electrophilicity, facilitating nucleophilic aromatic substitution reactions.
Tertiary Amine Functionality: The N-cyclohexyl-N-methyl group contributes to steric bulk and lipophilicity, influencing interactions with biological targets.
Salt Formation: Protonation of the amine group improves crystallinity and handling properties.
This compound’s molecular weight (~480.3 g/mol) and calculated logP (~3.5) suggest balanced solubility and membrane permeability, critical for both synthetic manipulation and bioactive studies.
2. Synthetic Pathways and Optimization
The synthesis of 2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride typically involves sequential halogenation and alkylation steps:
Bromination of 2-Aminobenzyl Derivatives: Selective dibromination at the 3- and 5-positions using reagents like N-bromosuccinimide (NBS) under controlled conditions.
Amine Alkylation: Reaction of the brominated intermediate with N-methylcyclohexylamine in the presence of a base (e.g., K₂CO₃) to form the tertiary amine linkage.
Salt Formation: Treatment with hydrochloric acid to yield the hydrochloride salt, followed by recrystallization for purification.
Recent advancements leverage microwave-assisted synthesis to reduce reaction times and improve yields (up to 72%). Additionally, catalytic methods employing palladium or copper complexes have been explored to enhance regioselectivity during bromination.
3. Pharmacological and Biological Applications
2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride has demonstrated significant potential in biomedical research:
Central Nervous System (CNS) Targeting: Preliminary studies indicate moderate affinity for serotonin receptors (5-HT₂C), suggesting utility in mood disorder therapeutics.
Antimicrobial Activity: The bromine atoms confer potent activity against drug-resistant Staphylococcus aureus (MIC: 2–4 μg/mL) by disrupting bacterial membrane integrity.
Enzyme Inhibition: In vitro assays reveal inhibitory effects on protein kinase C (PKC), positioning it as a candidate for anticancer drug development.
The hydrochloride salt formulation enhances bioavailability, with 85% stability in simulated intestinal fluid over 12 hours, a critical factor for oral administration.
4. Industrial and Material Science Relevance
Beyond pharmacology, 2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride finds utility in specialized applications:
Coordination Chemistry: Acts as a ligand for transition metal catalysts in cross-coupling reactions, leveraging its electron-deficient aromatic ring.
Polymer Additives: Incorporated into flame-retardant polymers due to bromine’s radical-scavenging properties.
Analytical Standards: Used as a reference compound in mass spectrometry and HPLC method development.
5. Challenges and Limitations
Despite its promise, the widespread adoption of 2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride faces hurdles:
Synthetic Complexity: Multi-step synthesis and purification processes escalate production costs.
Environmental Concerns: Brominated compounds raise ecotoxicity issues, necessitating stringent waste management protocols.
Metabolic Instability: Rapid hepatic clearance observed in preclinical models requires structural optimization for prolonged efficacy.
6. Future Prospects and Innovations
Ongoing research aims to expand the utility of 2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride through:
Prodrug Design: Masking the amine group to enhance metabolic stability and tissue targeting.
Nanocarrier Integration: Encapsulation in lipid-based nanoparticles to improve CNS delivery.
Sustainable Synthesis: Developing electrochemical bromination methods to reduce halogen waste.
2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine Hydrochloride embodies the convergence of synthetic ingenuity and multifunctional design. Its brominated aromatic system, coupled with a tailored amine scaffold, offers a versatile platform for drug discovery, catalysis, and material engineering. While challenges in scalability and environmental impact persist, innovations in synthetic methodologies and application-specific modifications hold the key to unlocking its full potential. As interdisciplinary research advances, this compound is poised to play a pivotal role in addressing complex scientific and industrial challenges.