- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Bromhexine Hydrochloride API
Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine Hydrochloride API
Cas#611-75-6
Send Inquiry
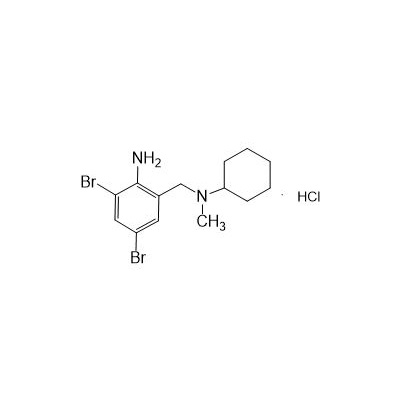
Formula

Chinese product name: Bromhexine hydrochloride
Chinese aliases: bromhexine hydrochloride; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N-methylbenzylamine hydrochloride; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloride;
English product name: Bromhexine Hydrochloride API
Cas#611-75-6
Molecular formula: C14H21Br2ClN2
Molecular weight: 412.6
Appearance and properties: white solid
Domestic registration number of API: Y20170001511
Usage: Used for acute and chronic bronchitis, asthma, bronchiectasis, and emphysema. It is especially suitable for people who have difficulty coughing up white sticky sputum and critical emergencies caused by extensive obstruction of small bronchi by sputum.
Bromhexine Hydrochloride API is for respiratory drug and cough with phlegm drug.
Bromhexine Hydrochloride API: A Comprehensive Overview
Introduction
Bromhexine Hydrochloride API (Active Pharmaceutical Ingredient) is a mucolytic agent widely recognized for its efficacy in managing respiratory disorders characterized by excessive mucus production. As a derivative of the alkaloid vasicine, Bromhexine Hydrochloride API works by reducing mucus viscosity, facilitating expectoration, and improving airway clearance. This article explores the pharmacological properties, therapeutic applications, manufacturing processes, and market dynamics of Bromhexine Hydrochloride API, emphasizing its significance in modern medicine.
Pharmacological Mechanism
Bromhexine Hydrochloride API exerts its mucolytic action by depolymerizing mucopolysaccharide fibers in bronchial secretions. It stimulates the production of serous fluid in the respiratory tract, enhancing the hydrolytic activity of enzymes such as hyaluronidase. Additionally, Bromhexine Hydrochloride API promotes the regeneration of alveolar surfactant, improving lung compliance. Its bioavailability and rapid absorption make it a preferred choice in formulations targeting chronic bronchitis, asthma, and other obstructive pulmonary diseases.
Therapeutic Applications
Clinically, Bromhexine Hydrochloride API is incorporated into syrups, tablets, and injectables to address acute and chronic respiratory conditions. Studies highlight its adjunctive role in treating COVID-19-related mucus retention, underscoring its versatility. The API’s ability to synergize with antibiotics by enhancing their penetration into lung tissues further broadens its therapeutic scope. Regulatory agencies worldwide have approved Bromhexine Hydrochloride API as a safe and effective mucolytic agent, solidifying its position in global pharmacopeias.
Manufacturing and Quality Control
The synthesis of Bromhexine Hydrochloride API involves multi-step chemical processes, starting from vasicine extraction or synthetic derivatization. Strict adherence to Good Manufacturing Practices (GMP) ensures high purity (>99%) and compliance with pharmacopeial standards (e.g., USP, EP). Advanced analytical techniques, including HPLC and NMR, are employed to validate the API’s identity, potency, and absence of impurities. Manufacturers prioritize sustainable practices to minimize environmental impact during Bromhexine Hydrochloride API production.
Market Dynamics
The global demand for Bromhexine Hydrochloride API is driven by rising respiratory disease prevalence and the expanding generics market. Asia-Pacific dominates production, with India and China accounting for over 60% of the supply. Patent expirations and cost-effective manufacturing have intensified competition, prompting innovation in formulation technologies. Bromhexine Hydrochloride API is projected to witness a CAGR of 4.8% from 2023 to 2030, reflecting its enduring relevance in healthcare.
Safety and Regulatory Considerations
Bromhexine Hydrochloride API exhibits a favorable safety profile, with adverse effects typically limited to mild gastrointestinal disturbances. Regulatory bodies, including the FDA and EMA, mandate rigorous preclinical and clinical testing to ensure efficacy and safety. Post-marketing surveillance programs continuously monitor real-world outcomes, reinforcing confidence in Bromhexine Hydrochloride API-based therapies.
Future Perspectives
Ongoing research explores novel applications of Bromhexine Hydrochloride API, such as nanoparticle-based delivery systems for targeted action. Collaborative efforts between academia and industry aim to optimize its pharmacokinetics and expand indications. As personalized medicine gains traction, Bromhexine Hydrochloride API is poised to remain a cornerstone in respiratory therapeutics.
Conclusion
Bromhexine Hydrochloride API exemplifies the intersection of traditional pharmacology and modern innovation. Its proven mucolytic efficacy, coupled with robust manufacturing frameworks and evolving applications, ensures its sustained impact on global health. As respiratory burdens escalate, Bromhexine Hydrochloride API will continue to play a pivotal role in improving patient outcomes worldwide.