- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
What Makes Tofacitinib Citrate a Reliable Pharmaceutical Option?
2025-09-18
Tofacitinib Citrate is a well-recognized active pharmaceutical ingredient (API) used in the treatment of autoimmune conditions. With the growing global demand for innovative therapies that can effectively manage chronic diseases, the interest in this compound has significantly increased. Jiangsu Run'an Pharmaceutical Co. Ltd. has been dedicated to the research, development, and manufacturing of high-quality pharmaceutical intermediates and APIs, ensuring that Tofacitinib Citrate produced under our name meets strict international quality standards.
In this article, we will discuss in detail the characteristics, parameters, importance, and applications of Tofacitinib Citrate. Additionally, you will find technical specifications, a structured overview of its benefits, and answers to some frequently asked questions to provide comprehensive insight into this product.
Why Is Tofacitinib Citrate Important?
The importance of Tofacitinib Citrate lies in its unique mechanism of action. It belongs to the Janus kinase (JAK) inhibitors family, which specifically targets pathways responsible for immune response regulation. This makes it particularly effective for autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.
Its pharmaceutical relevance is enhanced by:
-
Targeted mechanism: Reduces overactive immune responses while minimizing widespread immunosuppression.
-
Proven clinical use: Widely studied and approved in many global markets.
-
High therapeutic demand: Increasing prevalence of autoimmune diseases boosts long-term demand.
-
Reliable manufacturing: Strict compliance with GMP ensures consistent quality.
Product Parameters of Tofacitinib Citrate
Below is a technical summary of our Tofacitinib Citrate specifications. Jiangsu Run'an Pharmaceutical Co. Ltd. ensures that each batch complies with stringent quality and purity guidelines.
General Parameters
-
Product Name: Tofacitinib Citrate
-
Form: White to off-white crystalline powder
-
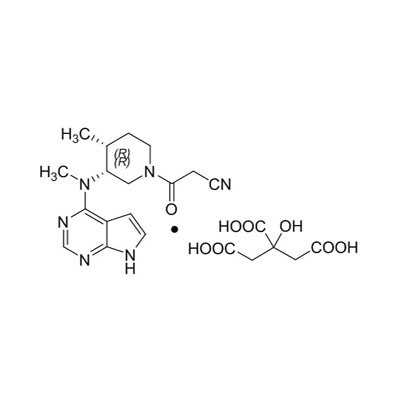
Molecular Formula: C₁₆H₂₀N₆O·C₆H₈O₇
-
Molecular Weight: 504.5 g/mol
-
CAS Number: 540737-29-9
-
Quality Standard: In-house / customized per client request
-
Packaging: Available in fiber drums, double-layer polyethylene bags, or customized solutions
Typical Technical Specifications
| Parameter | Specification |
|---|---|
| Appearance | White to off-white powder |
| Assay (HPLC) | ≥ 99.0% |
| Identification | Complies with standard references |
| Loss on Drying | ≤ 0.5% |
| Residual Solvents | Meets ICH Q3C guidelines |
| Heavy Metals | ≤ 10 ppm |
| Related Substances | ≤ 0.2% (single impurity) |
| Storage Condition | Store in a cool, dry, and dark place |
| Shelf Life | 24 months under recommended storage |
Key Advantages of Choosing Our Tofacitinib Citrate
When selecting a pharmaceutical supplier, both quality and reliability are crucial. Jiangsu Run'an Pharmaceutical Co. Ltd. ensures the following advantages for clients worldwide:
-
High Purity Levels – Achieving assays ≥ 99.0% ensures maximum efficiency in formulation.
-
Global Standards – Production facilities comply with cGMP guidelines and international norms.
-
Consistent Supply – Robust manufacturing capacity guarantees reliable delivery.
-
Customized Packaging – Flexible packaging solutions tailored to client logistics needs.
-
Technical Support – Dedicated support team provides detailed product documentation and COAs.
Applications of Tofacitinib Citrate
Tofacitinib Citrate is widely used across different therapeutic areas, with its primary role in:
-
Rheumatoid Arthritis – Helps relieve joint pain, swelling, and stiffness.
-
Psoriatic Arthritis – Improves physical function and reduces skin-related symptoms.
-
Ulcerative Colitis – Provides remission and maintenance therapy for chronic bowel inflammation.
-
Ongoing Research – Investigated for other autoimmune disorders, highlighting future potential.
The growing adoption of oral JAK inhibitors emphasizes the strategic role of Tofacitinib Citrate in the pharmaceutical industry.
Frequently Asked Questions about Tofacitinib Citrate
Q1: What is Tofacitinib Citrate mainly used for?
A1: Tofacitinib Citrate is primarily used as an active pharmaceutical ingredient in medications designed to treat autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. Its function as a JAK inhibitor allows it to control immune overreactions effectively.
Q2: How should Tofacitinib Citrate be stored to maintain its stability?
A2: It should be stored in a cool, dry, and well-ventilated environment, away from direct light and heat. Proper storage under controlled conditions ensures a shelf life of 24 months.
Q3: What quality standards does Jiangsu Run'an Pharmaceutical Co. Ltd. follow in producing Tofacitinib Citrate?
A3: Our facilities strictly comply with cGMP standards, while production is monitored under rigorous quality control systems. Each batch is tested for purity, assay, and safety parameters, with certificates of analysis provided to clients.
Q4: Can Jiangsu Run'an Pharmaceutical Co. Ltd. provide customized packaging or specific grades?
A4: Yes. We understand that each client has unique requirements, so we provide flexible packaging options and tailor-made specifications according to client project demands.
Why Partner with Jiangsu Run'an Pharmaceutical Co. Ltd.?
Jiangsu Run'an Pharmaceutical Co. Ltd. has established a reputation for delivering high-quality pharmaceutical ingredients to global partners. By focusing on quality assurance, customer service, and innovation, we ensure long-term cooperation and trust.
When you choose our Tofacitinib Citrate, you are choosing:
-
Consistency in quality and performance
-
International compliance and certifications
-
A reliable partner for supply chain stability
-
Professional support for all technical and documentation needs
Conclusion
Tofacitinib Citrate has become a cornerstone in the development of therapies targeting autoimmune diseases. Its effectiveness, combined with the precision of Jiangsu Run'an Pharmaceutical Co. Ltd.'s manufacturing standards, makes it a dependable choice for pharmaceutical companies worldwide.
For further information, technical documentation, or collaboration opportunities, please contact us directly.