- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
What Makes Apremilast API a Reliable Choice in Pharmaceutical Manufacturing?
2025-09-11
In the competitive pharmaceutical market, selecting a trusted source for active pharmaceutical ingredients (APIs) is crucial for ensuring drug safety, stability, and therapeutic performance. One such important product is Apremilast API, widely recognized for its role in treating inflammatory conditions. This article explores the specifications, benefits, and essential details of Apremilast API, alongside professional insights for pharmaceutical partners.
Understanding Apremilast API
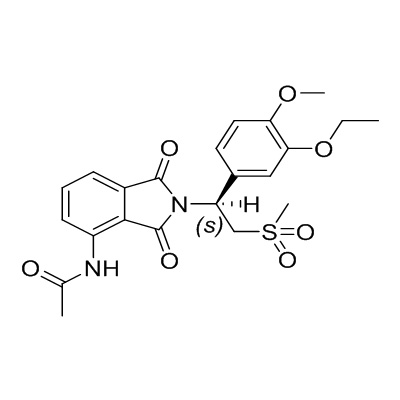
Apremilast API is a small-molecule inhibitor targeting phosphodiesterase 4 (PDE4), a key enzyme involved in inflammatory responses. Its mechanism reduces the production of pro-inflammatory cytokines, thereby providing therapeutic relief in autoimmune and inflammatory diseases. Manufacturers and research institutions rely on a consistent, high-quality Apremilast API for drug formulation.
Product Parameters of Apremilast API
To guarantee quality, pharmaceutical companies often look into clear technical parameters before sourcing APIs. Below is a structured overview of Apremilast API specifications:
Key Features:
-
Product Name: Apremilast API
-
Molecular Formula: C22H24N2O7S
-
Molecular Weight: 460.50 g/mol
-
CAS Number: 608141-41-9
-
Appearance: White to off-white powder
-
Purity (HPLC): ≥ 99%
-
Solubility: Freely soluble in dimethyl sulfoxide (DMSO), sparingly soluble in water
-
Storage Conditions: Store in a cool, dry, and well-ventilated area, away from light and moisture
-
Shelf Life: 24 months under recommended storage conditions
Technical Specifications Table:
| Parameter | Specification |
|---|---|
| Product Name | Apremilast API |
| CAS Number | 608141-41-9 |
| Molecular Formula | C22H24N2O7S |
| Molecular Weight | 460.50 g/mol |
| Appearance | White to off-white crystalline |
| Assay (Purity by HPLC) | ≥ 99% |
| Solubility | DMSO soluble, water sparingly |
| Storage Temperature | 2°C – 8°C |
| Shelf Life | 2 years |
Applications of Apremilast API
Apremilast API plays a central role in pharmaceutical formulations used for treating several chronic conditions:
-
Psoriasis: Reduces scaling, erythema, and plaque severity.
-
Psoriatic Arthritis: Provides long-term symptom control by lowering joint inflammation.
-
Behçet's Disease: Helps manage oral ulcers caused by inflammatory responses.
-
Other Autoimmune Disorders: Potentially beneficial in various off-label studies targeting chronic inflammation.
Why Choose Jiangsu Run'an Pharmaceutical Co. Ltd.?
When it comes to sourcing Apremilast API, consistency and compliance are non-negotiable. Jiangsu Run'an Pharmaceutical Co. Ltd. ensures:
-
Strict Quality Control: GMP-certified production facilities ensure pharmaceutical-grade consistency.
-
Regulatory Compliance: Products align with international pharmacopeia standards.
-
Scalability: From pilot-scale batches to large commercial quantities.
-
Technical Support: Dedicated experts provide formulation guidance and documentation support.
Benefits of Apremilast API
The selection of high-quality Apremilast API ensures not only effective formulation but also regulatory approval and patient trust.
Core Benefits:
-
High Purity: Guarantees reliable therapeutic efficacy.
-
Stable Formulation: Maintains potency throughout shelf life.
-
Flexible Application: Compatible with tablets and novel dosage forms.
-
Global Supply Readiness: Jiangsu Run'an Pharmaceutical Co. Ltd. supports international shipping with documentation.
Frequently Asked Questions About Apremilast API
Q1: What is Apremilast API used for?
A1: Apremilast API is primarily used in the treatment of psoriatic arthritis, psoriasis, and Behçet's disease. Its anti-inflammatory action targets cytokines responsible for chronic inflammatory responses, making it valuable for autoimmune therapies.
Q2: How is Apremilast API stored and handled?
A2: Apremilast API should be stored between 2°C and 8°C, in a tightly sealed container, away from direct sunlight and moisture. Proper handling includes using protective equipment and ensuring a controlled environment to maintain quality.
Q3: What makes Jiangsu Run'an Pharmaceutical Co. Ltd. a reliable supplier of Apremilast API?
A3: The company offers GMP-compliant production, robust quality control, and international regulatory support. Their long-standing expertise in pharmaceutical manufacturing makes them a preferred choice for global partners.
Q4: What is the purity level of Apremilast API supplied by Jiangsu Run'an Pharmaceutical Co. Ltd.?
A4: The product consistently achieves ≥99% purity by HPLC analysis, ensuring effective formulation performance and compliance with strict industry standards.
Conclusion
Pharmaceutical companies seeking a dependable partner for Apremilast API can rely on Jiangsu Run'an Pharmaceutical Co. Ltd. for consistent quality, compliance, and global supply capabilities. With precise product specifications and extensive industry expertise, Jiangsu Run'an continues to deliver trusted solutions for the development of therapies targeting inflammatory diseases.
For inquiries, partnerships, or detailed specifications, please contact Jiangsu Run'an Pharmaceutical Co. Ltd. directly.